Summary: In models of ALS and Huntington’s disease, exposure to cold temperatures actively removes protein clumps, thereby preventing protein aggregation.
Source: University of Cologne
Cold activates a cellular cleansing mechanism that breaks down harmful protein aggregations responsible for various diseases associated with aging. In recent years, studies on different model organisms have already shown that life expectancy increases significantly when body temperature is lowered.
However, precisely how this works is still unclear in many areas.
A research team at the University of Cologne’s CECAD Cluster of Excellence in Aging Research has now unlocked one responsible mechanism.
The study ‘Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes’ has appeared in Nature Aging.
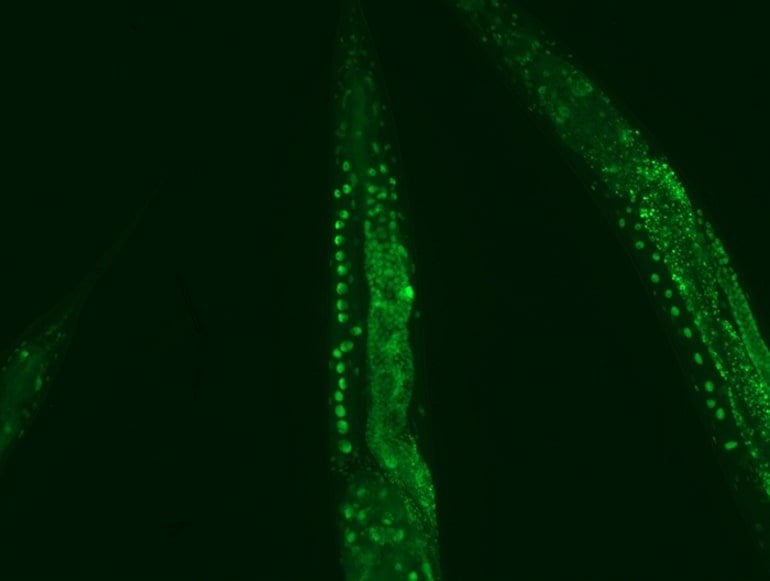
Professor Dr David Vilchez and his working group used a non-vertebrate model organism, the nematode Caenorhabditis elegans, and cultivated human cells. Both carried the genes for two neurodegenerative diseases which typically occur in old age: amyotrophic lateral sclerosis (ALS) and Huntington’s disease.
Both diseases are characterized by accumulations of harmful and damaging protein deposits – so-called pathological protein aggregations. In both model organisms, cold actively removed the protein clumps, thus preventing the protein aggregation that is pathological in both ALS and Huntington’s disease.
More precisely, the scientists explored the impact of cold on the activity of proteasomes, a cellular mechanism that removes damaged proteins from cells. The research revealed that the proteasome activator PA28γ/PSME3 mitigated the deficits caused by aging in both the nematode and in the human cells. In both cases, it was possible to activate proteasome activity through a moderate decrease in temperature.
“Taken together, these results show how over the course of evolution, cold has preserved its influence on proteasome regulation – with therapeutic implications for aging and aging-associated diseases,” said Professor Vilchez.
Aging is a major risk factor for several neurodegenerative diseases associated with protein aggregation, including Alzheimer’s, Parkinson’s, Huntington’s and ALS. Vilchez added: “We believe that these results may be applied to other age-related neurodegenerative diseases as well as to other animal species.”
A key finding was that the proteasome activity can also be increased by genetic overexpression of the activator. That way, disease-causing proteins can be eliminated even at the normal body temperature of 37 degrees Celsius. These results may provide therapeutic targets for aging and aging-associated diseases.
It has long been known that while extremely low temperatures can be harmful to organisms, a moderate reduction in body temperature can have very positive effects. For example, a lower body temperature prolongs the longevity of cold-blooded animals such as worms, flies or fish, whose body temperature fluctuates with the temperature of the environment.
However, the same phenomenon also applies to mammals, who maintain their body temperature within a narrow range no matter how cold or warm their environment is.
For example, the nematode lives much longer if it is moved from the standard temperature of 20 degrees Celsius to a colder temperature of 15 degrees Celsius. And in mice, a slight decrease in body temperature of just 0.5 degrees significantly extends their lifespan.
This supports the assumption that temperature reduction plays a central role in longevity in the animal kingdom and is a well-conserved evolutionary mechanism.
Even in humans, a correlation between body temperature and lifespan has been reported. Normal human body temperature is between 36.5 and 37 degrees Celsius. While an acute drop in body temperature below 35 degrees leads to hypothermia, human body temperature fluctuates slightly during the day and even reaches a cool 36 degrees during sleep.
Interestingly, a previous study reported that human body temperature has steadily decreased by 0.03 degrees Celsius per decade since the Industrial Revolution, suggesting a possible link to the progressive increase in human life expectancy over the last 160 years.
The research was conducted at the University of Cologne’s CECAD Cluster of Excellence in Aging Research.
About this aging and neuroscience research news
Author: Eva Schissler
Source: University of Cologne
Contact: Eva Schissler – University of Cologne
Image: The image is credited to David Vilchez and Hyun Ju Lee
Original Research: Open access.
“Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes” by David Vilchez et al. Nature Aging
Abstract
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Aging is a primary risk factor for neurodegenerative disorders that involve protein aggregation.
Because lowering body temperature is one of the most effective mechanisms to extend longevity in both poikilotherms and homeotherms, a better understanding of cold-induced changes can lead to converging modifiers of pathological protein aggregation.
Here, we found that cold temperature (15 °C) selectively induces the trypsin-like activity of the proteasome in Caenorhabditis elegans through PSME-3, the worm orthologue of human PA28γ/PSME3.
This proteasome activator is required for cold-induced longevity and improves age-related deficits in protein degradation. Moreover, cold-induced PA28γ/PSME-3 diminishes protein aggregation in C. elegans models of age-related diseases such as Huntington’s and amyotrophic lateral sclerosis.
Notably, exposure of human cells to moderate cold temperature (36 °C) also activates trypsin-like activity through PA28γ/PSME3, reducing disease-related protein aggregation and neurodegeneration. Q
Likewise, our findings reveal a beneficial role of cold temperature that crosses evolutionary boundaries with potential implications for multi-disease prevention.
